Alva Presbiteri (an external Ph.D. student of CSL) and co-workers are closing in on pinpointing the mechanisms behind the immune response triggered in patients undergoing open heart surgery. The team’s research reveals that by administering supplementary enzymes, so-called alkaline phosphates, to patients undergoing cardiac surgery, the latter can regain control over their own immune response. This in turn results in a surprisingly shorter recovery time and a considerably higher survival rate. The team’s results were recently published in the leading open-access journal Frontiers in Immunology.
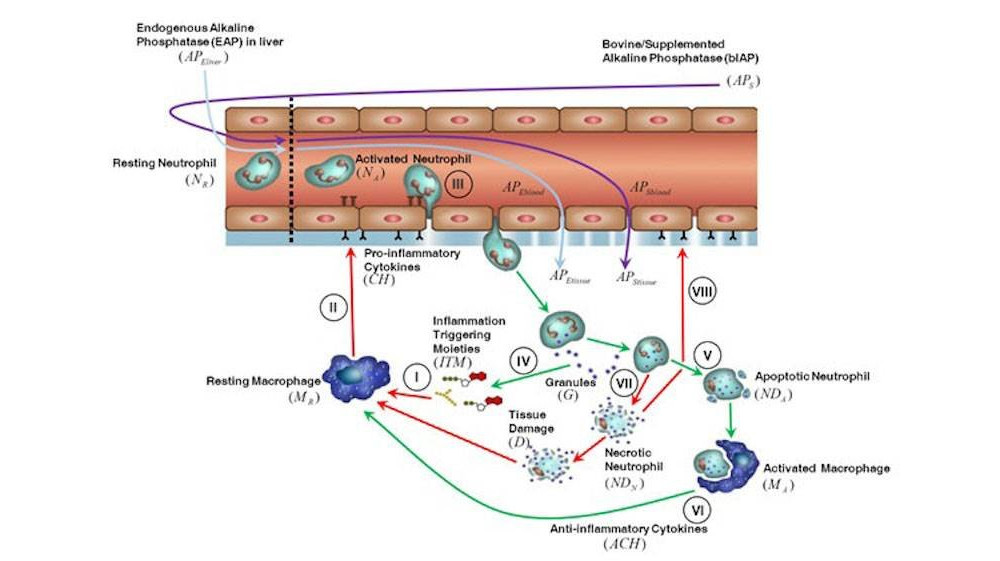
Alkaline phosphatase (AP) is an enzyme that exhibits anti-inflammatory effects by dephosphorylating inflammation triggering moieties (ITMs) like bacterial lipopolysaccharides and extracellular nucleotides. AP administration aims to prevent and treat peri- and post-surgical ischemia reperfusion injury in cardiothoracic surgery patients. Recent studies reported that intravenous bolus administration and continuous infusion of AP in patients undergoing coronary artery bypass grafting with cardiac valve surgery induce an increased release of liver-type “tissue non-specific alkaline phosphatase” (TNAP) into the bloodstream. The release of liver-type TNAP into circulation could be the body’s way of strengthening its defense against a massive ischemic insult. However, the underlying mechanism behind the induction of TNAP is still unclear. To obtain a deeper insight into the role of AP during surgery, Presbiteri et al. developed a mathematical model of systemic inflammation that clarifies the relation between supplemented AP and TNAP and describes a plausible induction mechanism of TNAP in patients undergoing cardiothoracic surgery. The model was validated against clinical data from patients treated with bovine Intestinal AP (bIAP treatment) or without AP (placebo treatment), in addition to standard care procedures. We performed additional in-silico experiments adding a secondary source of ITMs after surgery, as observed in some patients with complications, and predicted the response to different AP treatment regimens. Our results show a strong protective effect of supplemented AP for patients with complications. The model provides evidence of the existence of an induction mechanism of liver-type tissue non-specific alkaline phosphatase, triggered by the supplementation of AP in patients undergoing cardiac surgery. To the best of our knowledge this is the first time that a quantitative and validated numerical model of systemic inflammation under clinical treatment conditions is presented.
Link to the scientific article: click here.
Link to the UvA news item: click here.