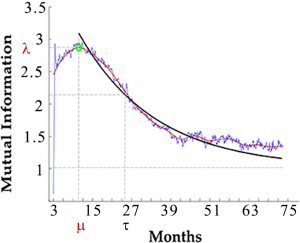
Mancini et al. predict a secondary phase in HIV progression characterized by intermediate susceptibility to temporary treatment, up to 10 months post-infection. This is in addition to the acute phase of about 1 month post-infection which is already known to be characterized by a high susceptibility to temporary treatments. However, this acute phase is often missed by the time the patient is diagnosed. This secondary phase of intermediate susceptibility, if verified, may change the way HIV is treated upon a new diagnosis.
Abstract:
It is still unclear under which conditions temporary combined antiretroviral therapy (cART) results in a prolonged remission after interruption. Clinical trials have contradicting reposts about the effect of cART during primary HIV infection on the disease progression. Here we propose that the apparent contradiction is due the presence of a window of opportunity for cART treatment observed in the in silico studies. We study non-linear correlations in the HIV dynamics over time using information theory. This approach requires a large dataset of CD4+ T lymphocytes and viral load concentrations over time. Since it is unfeasible to collect the required amount of data in clinical trials we use C-ImmSim, a clinically validated in silico model of the HIV infection, to simulate the HIV infection and temporary cART in 500 virtual patients for a period of 6 years post infection in time steps of 8 hours. We validate the results of our model with two published clinical trials of temporary cART in acute infection and analyse the impact of cART on the immune response. Our quantitative analysis predicts a “window of opportunity” of about ten months after the acute phase during which a temporary cART has significantly longer-lasting beneficial effects on the immune system as compared to treatment during the chronic phase. This window may help to explain the controversial outcomes of clinical trials that differ by the starting time and duration of the short-term course cART and provides a critical insight to develop appropriate protocols for future clinical trials.
Link: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0200892