P. S. Zun, A. J. Narracott, C. Chiastra, J. Gunn and A. G. Hoekstra have published a paper presenting a detailed location-specific validation methodology for in silico restenosis models. https://link.springer.com/article/10.1007/s13239-019-00431-4
Coronary artery restenosis is an important side effect of percutaneous coronary intervention. Computational models can be used to better understand this process. We report on an approach for validation of an in silico 3D model of in-stent restenosis in porcine coronary arteries and illustrate this approach by comparing the modelling results to in vivo data for 14 and 28 days post-stenting.
This multiscale model includes single-scale models for stent deployment, blood flow and tissue growth in the stented vessel, including smooth muscle cell (SMC) proliferation and extracellular matrix (ECM) production. The validation procedure uses data from porcine in vivo experiments, by simulating stent deployment using stent geometry obtained from micro computed tomography (micro-CT) of the stented vessel and directly comparing the simulation results of neointimal growth to histological sections taken at the same locations.
Metrics for comparison are
per-strut neointimal thickness and per-section neointimal area. The
neointimal area predicted by the model demonstrates a good agreement
with the detailed experimental data.
The approach presented here provides a very detailed, location-specific, validation methodology for in silico restenosis models. The model was able to closely match both histology datasets with a single set of parameters. Good agreement was obtained for both the overall amount of neointima produced and the local distribution. It should be noted that including vessel curvature and ECM production in the model was paramount to obtain a good agreement with the experimental data.
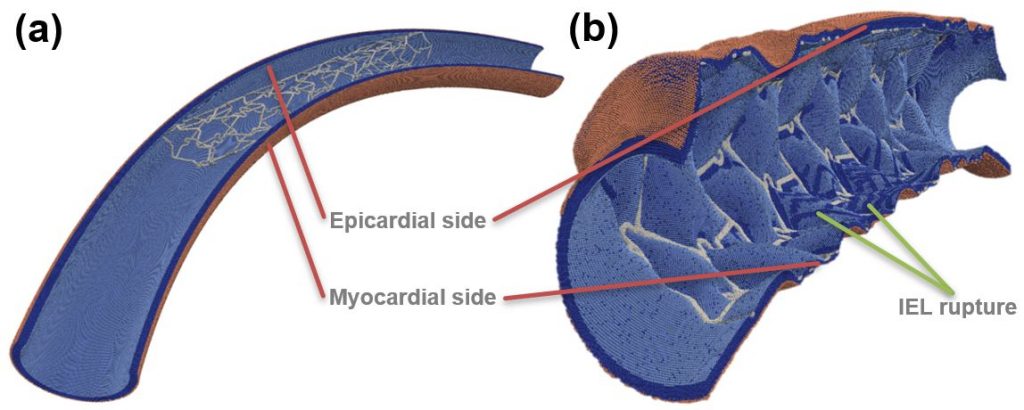
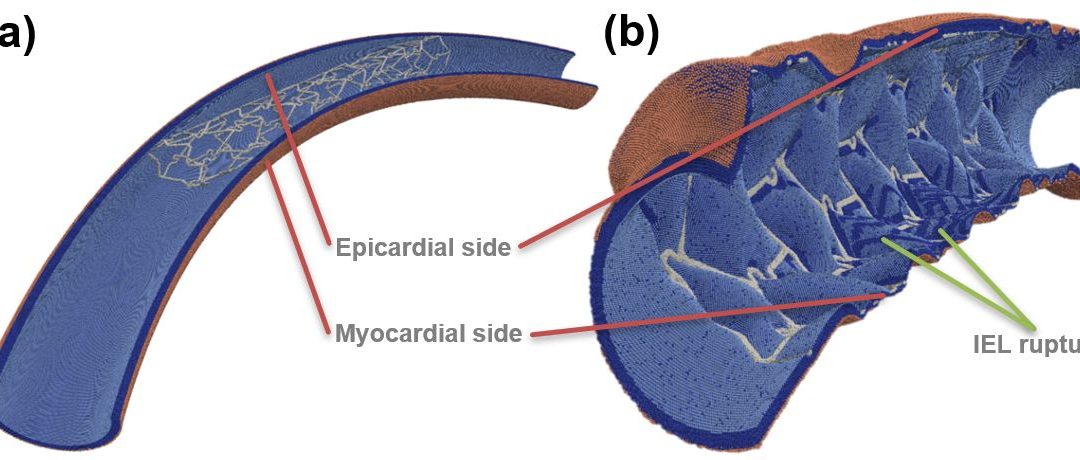
Figure: Toroidal vessel segment (a) pre- and (b) post-deployment. Internal elastic lamina (IEL) ruptures are visible on the inner curve, as well as near some struts. SMCs—dark blue, IEL—light blue, external elastic lamina—beige, stent—light grey.